PERI-STRIPS

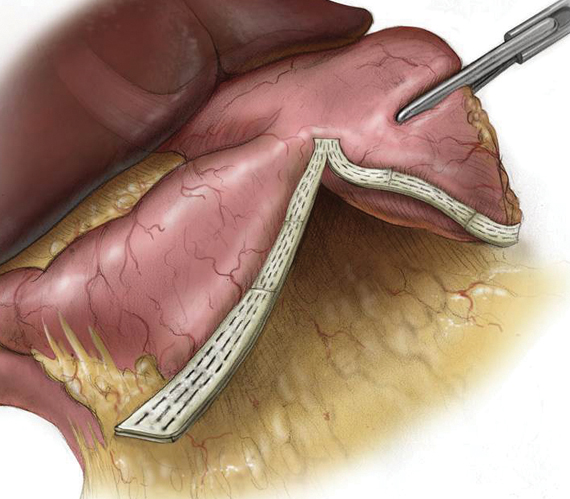
Confidence at the Staple Line
Over 20 years and 2.2 million implants later, PERI-STRIPS DRY with VERITAS is proven to improve clinical outcomes and reduce procedural complications.1,2,3
Strong From the Start (Burst Strength)*
An experimental study evaluating staple line seam integrity at intraluminal pressures in the immediate postoperative period with a canine model demonstrated:
Regardless of the operative techniques, the buttressed ileocolic anastomoses showed higher bursting pressures.*,8
Buttressed anastomoses never ruptured at the staple line.*,8
*Pre-clinical data results may not correlate to results in humans.
Additional Product Benefits
Outperforms Synthetic PGA Material
PERI-STRIPS DRY with VERITAS is derived from bovine pericardium and outperforms synthetic PGA material at the staple line over time in sleeve gastrectomy/gastric bypass patients.2
Reduced Risk of Staple Line Leaks and Bleeds
In a multicenter comparative use study, PERI-STRIPS DRY with VERITAS significantly lowered the risk of postoperative bleeding and leaks at the staple line compared to competitive products, over-sewing, and no staple line reinforcement.3
Fully Remodelable with Tissue Integration
Remodeling of PERI-STRIPS DRY with VERITAS entails two simultaneous processes that occur during normal wound healing: degradation of the VERITAS scaffold and deposition of new tissue and blood vessels, as demonstrated in pre-clinical studies*.4,5,6
*Pre-clinical data results may not correlate to results in humans.
Indications and Important Risk Information
INDICATIONS FOR USE
PERI-STRIPS DRY with VERITAS Collagen Matrix (PSDV) Reinforcement is intended for use as a prosthesis for the surgical repair of soft tissue deficiencies using surgical staplers when staple line reinforcement is needed.
(Linear reinforcement only) PSD-V can be used for reinforcement of staple lines during bariatric and gastric surgical procedures, inclusive of small bowel.
IMPORTANT RISK INFORMATION
Do not re-sterilize. Resterilization may cause changes to the tissue and negatively impact functionality of the device.
Discard all open unused components. Do not use product if there is damage to the pouch or seals.
Ensure the staple line is completely covered with the buttress or inadequate coverage after firing may result.
Do not use PSDV Reinforcement if the product has been exposed (1) to solutions above room temperature, (2) to chemicals, antibiotics, or other substances other than specifically addressed in the instructions, (3) or if heat indicator has been activated.
Use care when removing loading unit components from the stapler to prevent buttress dislodgement.
Do not get the buttress wet before applying Gel, or the buttress may not adhere to the stapler properly.
Ensure the anvil and cartridge sides of the loading unit are on the corresponding stapler jaws or the buttress may not adhere to the stapler properly. The cartridge and anvil sides of the PSDV Reinforcement loading unit differ; substitution of one side for the other may interfere with alignment and adherence of the buttress strips.
Final tissue compression, including PSDV Reinforcement, must meet the range specified by the stapler manufacturer; this is especially important if staple firings are overlapped. PSDV Reinforcement increases the total thickness of the area stapled by 0.4 mm – 1.2 mm (0.016” – 0.048”).
Rx Only. For safe and proper use of this device, refer to the Instructions for Use.